The ozone layer is a layer in Earth's atmosphere that absorbs most of the Sun's UV radiation. It contains relatively high concentrations of ozone, although it is still very small with regard to ordinary oxygen. Ozone is a gas made up of three oxygen atoms (O3). It occurs naturally in small amounts in the upper atmosphere (the stratosphere). Ozone protects life on Earth from the Sun's ultraviolet (UV) radiation. Ninety percent of the ozone in the atmosphere sits in the stratosphere, the layer of atmosphere between about 10 and 50 kilometers altitude.
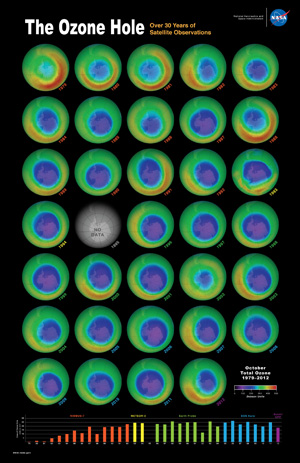
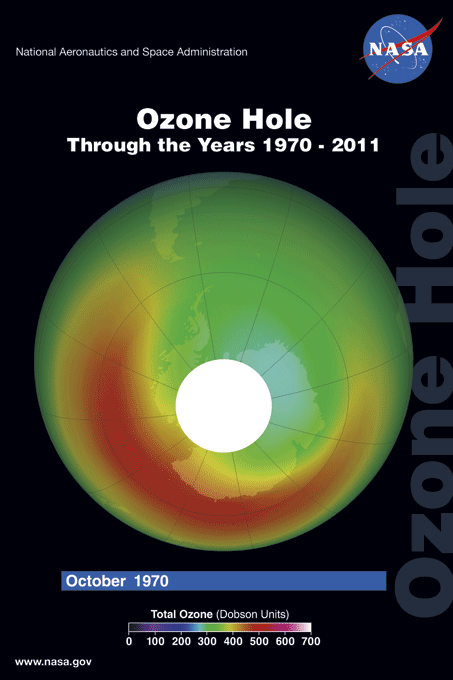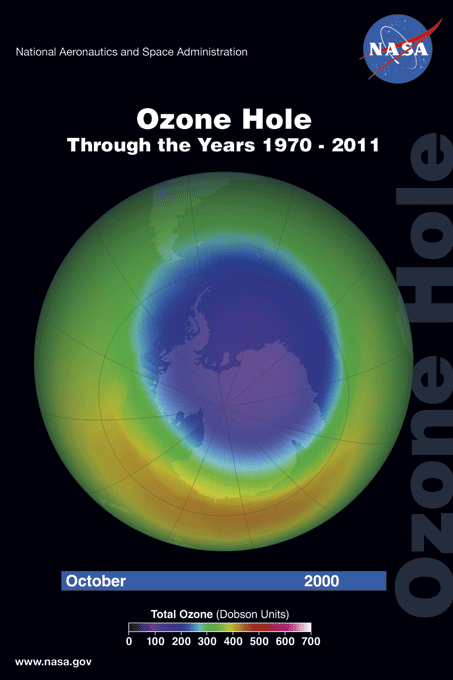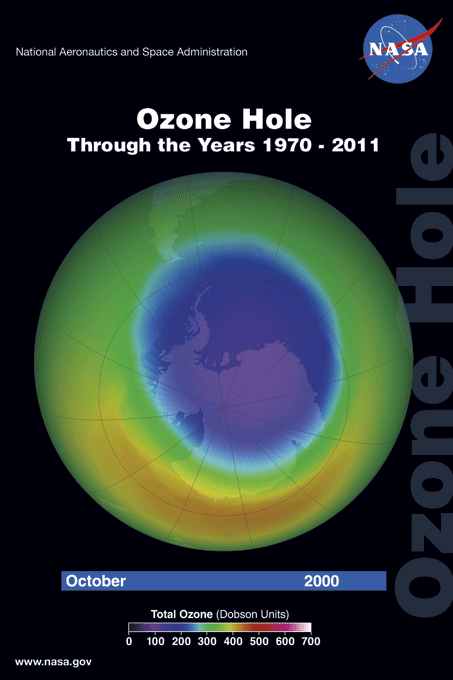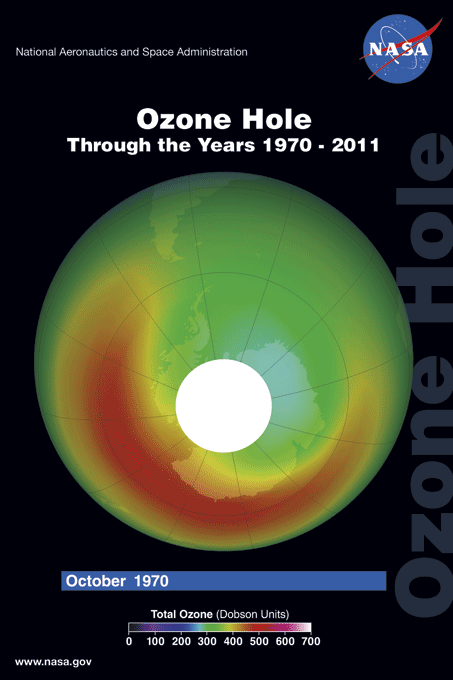
The ozone hole is a loss of stratospheric ozone over Antarctica. The ozone hole area is defined as the size of the region with total ozone below 220 Dobson units (DU). Dobson Units are a unit of measurement that refer to the thickness of the ozone layer in a vertical column from the surface to the top of the atmosphere, a quantity called the "total column ozone amount." Prior to 1979, total column ozone values over Antarctica never fell below 220 DU. The hole has been proven to be a result of human activities--the release of huge quantities of chlorofluorocarbons (CFCs) and other ozone depleting substances into the atmosphere.
For living creatures on Earth, there is good ozone and bad ozone. Good ozone is found in the stratosphere, far above the Earth's surface. At that height, it absorbs and scatters ultraviolet (UV) radiation from the Sun, particularly the most dangerous UV-B and UV-C forms. Ozone is the planet's natural sunscreen. Plants, animals, and all forms of life developed under a sky that shielded them from damaging and mutating radiation.
Whether the molecule is helpful or harmful has nothing to do with the chemical makeup and everything to do with location. About 10% of the ozone in the atmosphere is found in the troposphere, the layer we live in. This ozone is created by chemical reactions between air pollutants from vehicle exhaust, gasoline vapors, and other emissions. At ground level, high concentrations of ozone are toxic to people and plants.